Guide In Medical, a medical device startup, which operates as part of the NGT3 Technological Incubator based in Nazareth Israel, has announced receiving FDA market approval for its innovative IRRIS device. The device facilitates performing intubation, an essential and routine medical procedure in which a tube is inserted into the trachea to help open the patient’s airway.
Guide In Medical is currently in a second financing round to raise $2 million. The Company is poised for setting up distribution systems in Europe, having received CE Marking approval in 2017, and now in the U.S. upon receiving FDA approval. Guide In Medical has so far raised NIS 5 million from CTS, the pharmaceutical, medical equipment and veterinary company, which has joined the Company as a strategic partner, as well as from NGT3 and private investors.
The IRRIS was also approved by the Israel Ministry of Health (MOH) and is currently being tested by the intensive care unit of Hadassah Hospital, Mount Scopus. The target audience of the device is physicians in medical centers, paramedics in mobile intensive care units, the army, the police, and other organizations that manage intensive care units.
Intubation is a vital and common medical procedure performed under general anesthesia in operating rooms and sometimes also in field conditions. This is the third most common medical procedure in the U.S., and more than 100 million intubation procedures are performed each year worldwide. The procedure of inserting a tube into the trachea to ventilate the patient requires a high level of skill, maximum precision and speed for quick insertion, as the patient cannot breathe while the procedure is performed. Damage caused by faulty intubation, due to lack of experience of the doctor or paramedic, environmental conditions and/or secretions in the pharynx that obstruct the lungs, and sometimes a different anatomical structure of the patient, may be critical and cost the patient’s life.
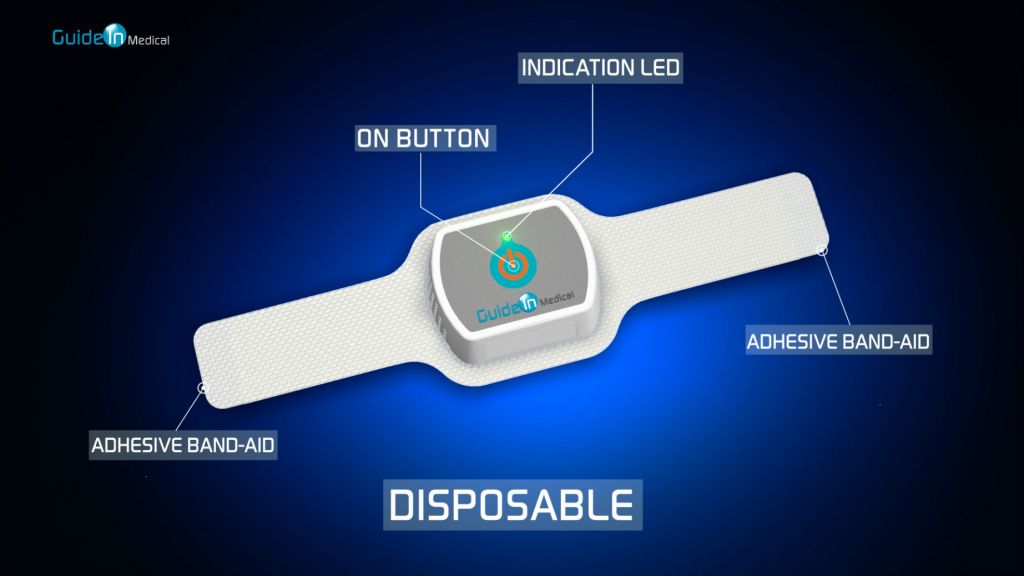
The solution is a non-invasive device, which is attached as a patch to the patient’s neck and transmits light of a specific wavelength into the tissues, lighting the inside of the throat. The special light, which blinks clearly, makes it easier for the medical staff to locate the trachea using a video laryngoscope (a device with a video camera) and can more easily guide the insertion of the ventilation tube during intubation.
Ariel Shrem, Co-Founder and CEO, Guide In Medical: “The advantages of the device lie in the simplicity of its operation and the ability to quickly and safely identify the trachea even in complicated situations. This is demonstrated by the results of the clinical trials and the positive feedback we received from physicians who have already tried the device. I believe that in the next few years the product will be used in every ambulance, operating room and battalion aid station. FDA approval is an important step in reaching the global market and in recruiting partners for distribution and of course additional investors as part of our growth plan.”
Zohar Gendler, CEO of NGT3: “Guide In Medical has succeeded in a short time and with maximum efficiency to complete development, conduct three clinical trials, publish two scientific articles, obtain regulatory approvals for marketing in the US, Europe and Canada, and bring on a strategic partner. The company is now turning to its next task of organizing for marketing and going to market.”
Guide In Medical, a medical device startup, was founded in 2015 based on a collaboration between the entrepreneurs, Itai Hayut, director and technological consultant, Ariel Shrem, CEO of the Company, and Dr. Elchanan Fried, head of the Intensive Care Unit at Hadassah Medical Center on Mt. Scopus and medical consultant of the Company.
Guide In Medical has developed an Infrared Red Intubation System (IRRIS) device that facilitates intubation, a vital and common medical procedure in which a tube is inserted into the trachea to ventilate the patient.
The technology was created as part of the Hebrew University’s Biomedical Entrepreneurship Program in collaboration with Hadassah Medical Center. The Company received an exclusive license to develop the technology from Yissum Research Development Company, the technology transfer company of the Hebrew University of Jerusalem, and Hadasit Medical Research Services & Development Ltd., the company for commercialization of technologies of Hadassah Medical Organization in Jerusalem.




