
August 9, 2023 – A new, and detailed analysis of early mother and fetus dynamics provides a greater understanding of gestational age (pregnancy stage) on maternal and fetal health, according to researchers at the Hebrew University of Jerusalem (HU) and Stanford University.
Discoveries from this research could fill a knowledge gap regarding normal placenta development that could improve maternal and fetal outcomes, and lead to advancements in treating placenta-related obstetric complications, such as preeclampsia (a high blood pressure disorder) and preterm birth.
The study, published in Nature, utilizes a detailed map of the interactions between the mother and fetus in the early stages of pregnancy to show how immune cells change to support a developing fetus and prevent harmful reactions.
“This research represents a major leap forward in our understanding of the maternal-fetal interface,” said lead researcher Dr. Shirley Greenbaum, Department of Obstetrics and Gynecology, Hadassah-Hebrew University Medical Center and Department of Pathology, Stanford University.
“We can use data captured to unravel the complex interactions between foreign fetal cells and maternal immune cells, and tackle puzzling questions such as ‘Why maternal immune cells are not attacking foreign fetal cells?’ and ‘What drives the dramatic changes we see in the structure of maternal vessels?’”
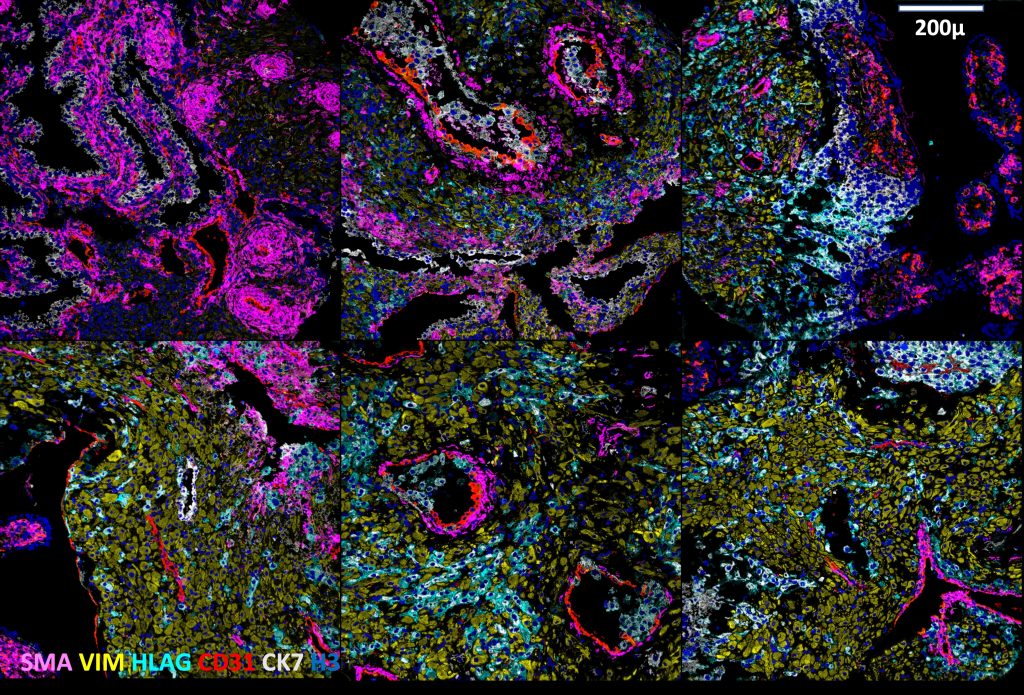
Using Multiplexed Ion Beam Imaging by time-of-flight (MIBI-TOF), the research team examined approximately 500,000 cells and 588 arteries in the intact decidua, which is the border between the maternal and fetal sides in the placenta. This approach, which enables detection of up to 40 markers simultaneously at the single cell level, allowed them to get a detailed understanding of the changes happening in the tissue at different stages of pregnancy.
They discovered that over time, unique, changing patterns help fetal cells interact with maternal blood vessels without triggering an immune response, which allows the fetus to receive necessary nutrients and oxygen without facing rejection from the mother’s immune system.

The researchers also found that fetal cells drive spiral artery remodeling (SAR). During normal pregnancy, maternal spiral arteries, which are normally coiled and constricted, dilate extensively to become wide, flaccid vessels that can transfer low velocity, low pressure blood flow to the placenta. The research team is now working on implementing these findings to examine samples from preeclamptic pregnancies, where the spiral artery remodeling process does not occur smoothly.
The research team includes:
- Shirley Greenbaum, Department of Obstetrics and Gynecology, Hadassah-Hebrew University Medical Center and Department of Pathology, Stanford University
- Erin Soon, Department of Pathology and the Immunology Program at Stanford University
- Noah F. Greenwald, Department of Pathology, and the Cancer Biology Program at Stanford University
- Inna Averbukh, Alex Baranski, Adam Kagel, Marc Bosse, Zumana Khair, Shirley Kwok, Shiri Warshawsky, Hadeesha Piyadasa, Mako Goldston, Angie Spence, Matt van de Rijn & Michael Angelo, Department of Pathology, Stanford University
- Gabrielle Rizzuto, Department of Pathology, University of California San Francisco
- Eleni G. Jaswa, Department of Obstetrics Gynecology and Reproductive Sciences, University of California San Francisco
- Geneva Miller, Morgan Schwartz, Will Graf & David Van Valen, Division of Biology and Bioengineering, California Institute of Technology
- Virginia D. Winn, Department of Obstetrics and Gynecology, Stanford University
- Travis Hollmann, Department of Pathology, Memorial Sloan Kettering Cancer Center, New York
- Leeat Keren, Department of Molecular Cell Biology, Weizmann Institute of Science




