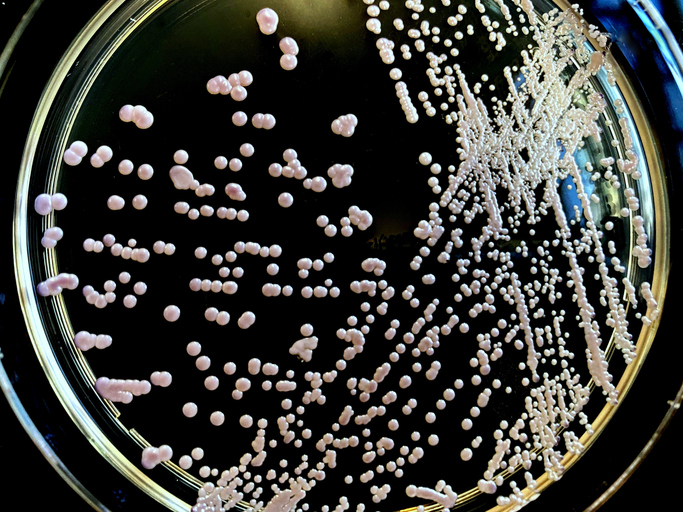
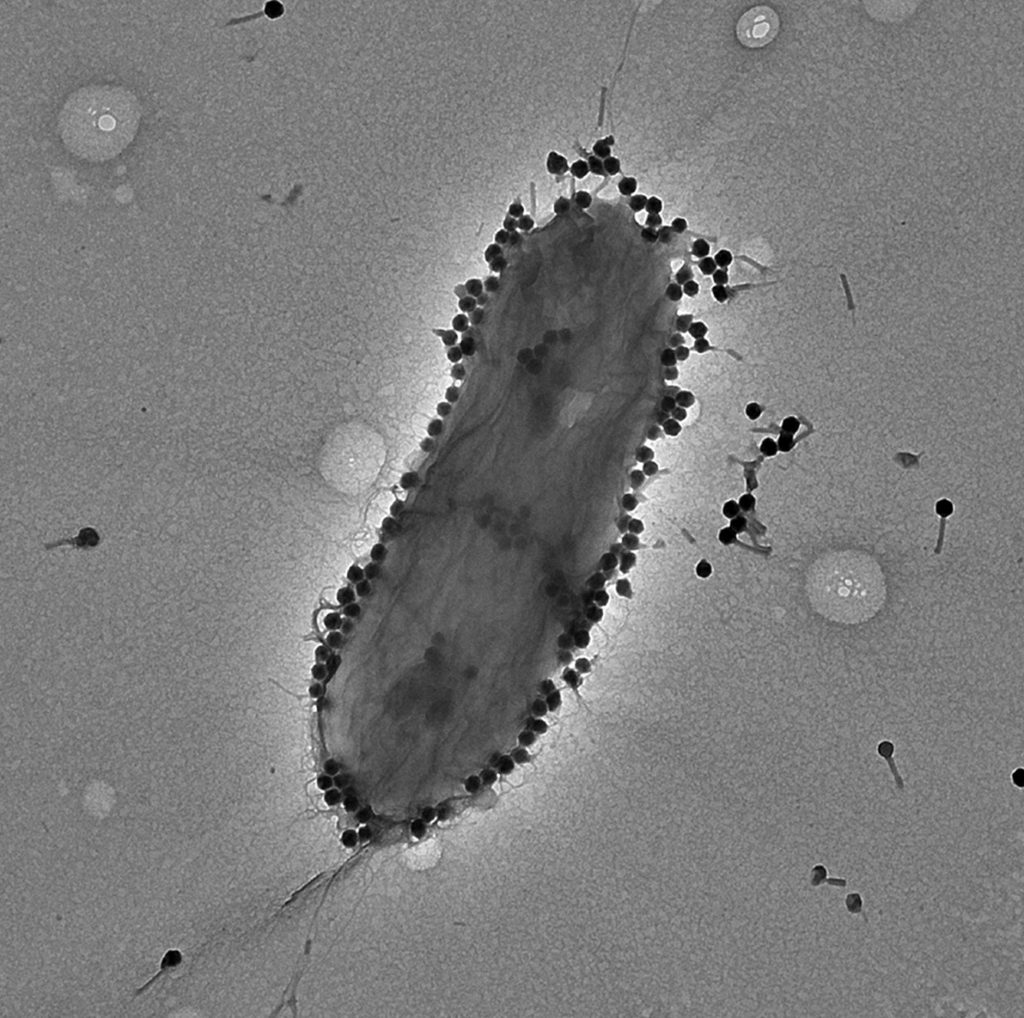
August 18, 2023 — A new international study using phage PASA16 on a compassionate basis to treat tough Pseudomonas aeruginosa infections (which can cause infections in the blood, lungs [pneumonia], or other parts of the body after surgery) has shown promising results, with an 86.6% success rate. Conducted by the Israeli Phage Therapy Center (IPTC) of Hadassah–Medical Center (HHUMC) and led by Prof. Ran Nir-Paz of the HHUMC Department of Clinical Microbiology and Infectious Diseases and IPTC as well as Ronen Hazan of the IPTC and the Institute of Biomedical and Oral Research (IBOR) in the Faculty of Dental Medicine at the Hebrew University of Jerusalem (HU), the research represents the largest study of its kind and demonstrates the potential effectiveness of PASA16 phage therapy in tackling challenging Pseudomonas aeruginosa infections. The success of the study paves the way for future clinical trials and provides encouragement for further exploration of phage therapy as an alternative and auxiliary approach against antibiotic-resistant infections.
Pseudomonas aeruginosa is a type of bacteria commonly found in soil, water, and plants, and as part of bacteria residing in humans. It is both a pathogen and opportunistic bacteria, causing infections in individuals with weakened immune systems. The infections can range from mild to severe, affecting various body parts, including the lungs, urinary tract, skin, and wounds. Known for its potentially complicated and life-threatening infections, Pseudomonas aeruginosa poses challenges in healthcare settings.
As the fight against antibiotic resistance continues, Phage therapy has re-emerged as a potential solution for antimicrobial-resistant and non-resolving infections, since phages specifically target other microorganisms. While compassionate use cases for phage therapy have been implemented, clinical trials remain limited. The IPTC study sheds light on the potential role of phages in combination with antibiotics in combating the hard-to-treat pathogen Pseudomonas aeruginosa infections that were unresponsive to conventional treatments.
All sixteen patients in the study were treated after demonstrating the susceptibility of their infective agent to both the phage alone and in combination with antibiotics. During the PASA16 phage treatment, only minor, manageable side effects were observed. Remarkably, 13 out of 15 patients with available data achieved a favorable clinical outcome. This highlights the potential of combining PASA16 phage with antibiotics as a promising approach for patients with previously unsuccessful treatments. The duration of treatment spanned from 8 days to 6 weeks (majority of ~ 2 weeks), with one- to twice-daily regimens, offering a time-efficient option. The phage, provided pro bono by the American phage company Adaptive Phage Therapeutics, was administered through various methods, including intravenous, local application to the infection site, and topical use.
Prof. Ran Nir-Paz expressed excitement about the findings, stating, ”We are elated by the promising results of our study using phage PASA16 to treat tough Pseudomonas aeruginosa infections. This groundbreaking research offers hope for patients with persistent infections and highlights the potential of phage therapy as a valuable alternative to conventional antibiotics in combating antibiotic-resistant pathogens.”
Prof. Ronen Hazan the co-lead researcher of the IPTC, also the head of the Bio-research institute of the Faculty of Dental Medicine at the Hebrew University stated: “We are encouraged by the findings! The study’s 86.6% success rate offers hope.”
The study was funded in part by The Israeli Science Foundation IPMP (ISF_1349/20), Rosetrees Trust (A2232), United States-Israel Binational Science Foundation (2017123), and the Milgrom Family Support Program.
For more information and a complete list of researchers, please click “Refractory Pseudomonas aeruginosa infections treated with phage PASA16: A compassionate use case series” published at MED.




