June 14, 2024 — A new study led by Dr. Milan Patra along with Professors Ittai Ben-Porath and Yuval Dor from the Faculty of Medicine of the Hebrew University of Jerusalem has revealed that senescent human pancreatic beta cells, cells which play a crucial role in insulin production, exhibit enhanced functional maturation through chromatin reorganization. Additionally, the study found that these cells show increased activity of the interferon pathway, which stimulates the immune system. This finding may offer a potential new avenue for tackling type I diabetes.
Background: The Diabetes Challenge
Diabetes, characterized by insulin deficiency or resistance, hinges on dysfunctional pancreatic beta cells, which are responsible for secreting insulin to remove glucose from the blood. Enhancing or preserving the function of these cells is pivotal for developing diabetes treatments. Globally, an estimated 463 million adults (roughly 1 in 11) grapple with this condition, a figure expected to balloon due to aging populations, urbanization, poor diets, and sedentary lifestyles. Projections indicate that by 2045, over 700 million people could be afflicted, posing daunting challenges to healthcare, economies, and public health efforts. Urgent action is imperative to stem this tide, necessitating effective prevention strategies, better access to care, and innovative treatments.
Key Findings: Functionality and Immune Response
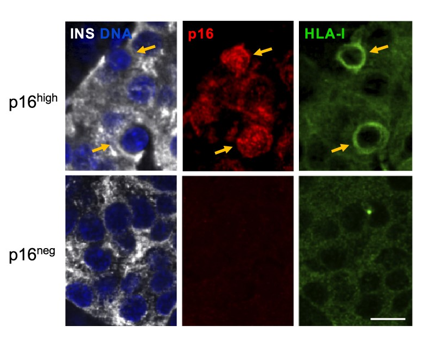
The study, published in Nucleic Acids Research, demonstrates that a significant portion of adult human pancreatic beta cells activate a gene called p16, which indicates that they are in an aging-like state, termed cellular senescence. Interestingly, these senescent cells, rather than showing signs of dysfunctionality, showed elevated levels of genes that are important for their function. Thus, these cells appear to possess a higher level of functionality and maturity compared to their non-senescent neighbors. This is surprising, as previously identified senescent cells in other tissues are generally thought to be dysfunctional and have harmful effects.
By analyzing the gene organization of senescent beta cells, the researchers discovered that they change the packaging of the genes—the chromatin, generating a reprogrammed organization that allows activation of functionality. Because of this, it appears that the aging beta cells have the ability to release insulin in response to glucose in even larger amounts, which helps regulate blood sugar levels effectively.
This study also found that senescent beta cells have elevated levels of genes that communicate with the immune system. This response, termed the “interferon response,” normally acts to indicate a viral infection to immune cells, recruiting their attack. However, the senescence beta cells activate this pathway in the absence of such infection: it is molecular changes in the cells themselves that simulate this response. The potential consequence is increased stimulation of immune cells to attack beta cells, the fundamental process that drives type I diabetes. This means that aging beta cells might help regulate immune responses and could be important for understanding autoimmune reactions in type 1 diabetes. Potentially, blocking this response, or the process of senescence, could be used to prevent the progression of type I diabetes in its early stages.
Implications for Diabetes Treatment
The discovery that aging pancreatic beta cells can retain high functionality and respond to immune signals challenges the traditional view that senescent cells are purely detrimental. This new understanding opens the door to potential therapies aimed at preserving or enhancing the insulin-secreting function of beta cells in diabetic patients.
“These findings are pivotal because they suggest that senescent beta cells are not a liability but may act, in a pre-designed manner, to improve insulin production as we age, countering other detrimental effects,” said Professor Ittai Ben-Porath. “Furthermore, if it will be further established that senescence of beta cells is a prominent feature of the early stages of type I diabetes, targeting of these cells through drug treatment could represent a novel approach for preventing autoimmune attack of beta cells.”
Future Research Directions
Future research plans include delving deeper into the mechanisms driving the increased activity of functional-maturation programs in aging beta cells, influenced by chromatin dynamics. A comprehensive understanding of these processes holds promise for the development of targeted therapies aimed at enhancing beta-cell functionality and lifespan, thereby improving the quality of life for individuals grappling with diabetes. Understanding how the process of senescence affects the interaction of immune cells with beta cells, and whether this is indeed associated with diabetes, may open the door for new treatment approaches.
The research paper titled “Senescence of human pancreatic beta cells enhances functional maturation through chromatin reorganization and promotes interferon responsiveness” is now available here.
Researchers:
Milan Patra1, Agnes Klochendler1, Reba Condiotti1, Binyamin Kaffe2, Sharona Elgavish3, Zeina Drawshy1, Dana Avrahami1, Masashi Narita4, Matan Hofree5 6, Yotam Drier5, Eran Meshorer2, Yuval Dor1, Ittai Ben-Porath1
Institutions:
- Department of Developmental Biology and Cancer Research, Institute for Medical Research Israel-Canada, Faculty of Medicine, The Hebrew University of Jerusalem
- Department of Genetics, the Institute of Life Sciences and the Edmond and Lily Safra Center for Brain Sciences (ELSC), The Hebrew University of Jerusalem
- Info-CORE, Bioinformatics Unit of the I-CORE at the Hebrew University of Jerusalem
- Cancer Research UK Cambridge Institute, Li Ka Shing Centre, University of Cambridge
- The Lautenberg Center for Immunology and Cancer Research, Institute for Medical Research Israel-Canada, Faculty of Medicine, The Hebrew University of Jerusalem
- School of Computer Science and Engineering, The Hebrew University of Jerusalem
Funding
Stichting Onderzoek Nederland (Y. Dor and I.B.-P.); Israel Science Foundation Legacy Heritage Program [1245/16 to I.B.-P.]; British Council BIRAX Program [65BX18MNIB to M.N. and I.B.-P.];Juvenile Diabetes Research Fund [3-SRA-2024-1479-S-B to Y. Dor and I.B.-P.]; Cooperation Program in Cancer Research of the Deutsches Krebsforschungszentrum (DKFZ) and Israel’s Ministry of Science, Technology and Space (MOST) [0004062 to I.B.-P.]; Human Islet Research Network [HIRN, U01 DK135001] and NIDDK [R01 DK133442 to Y. Dor]; Human Islet Research Network [HIRN, U01 DK134995 to D.A.]; Cancer Research UK Cambridge Institute Core Grant [C9545/A29580 to M.N.]; I.B.-P. holds the Woll Sisters and Brothers Chair in Cardiovascular Diseases; Y. Dor holds the Walter and Greta Stiel Chair and Research Grant in Heart studies. Funding for open access charge: Woll Sisters and Brothers Chair in Cardiovascular Diseases.